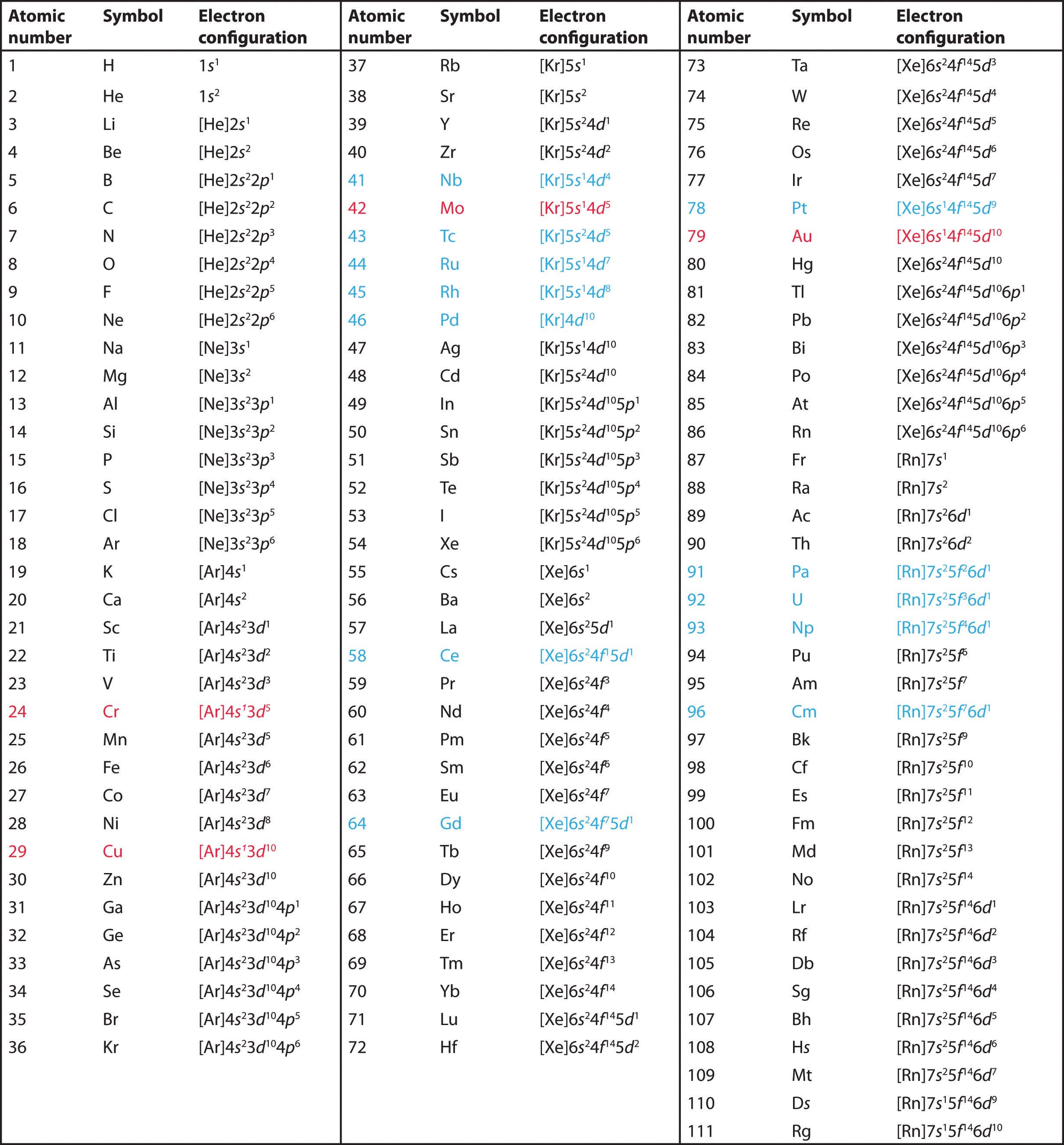
In this article at sidclasses, we will delve into the fascinating world of the atom, the fundamental building block of matter. Our journey will take us through Chapter 2 Structure Of Atom Class 11 Notes, its historical discovery, and the development of atomic models based on NCERT class 11. We will explore the structure of the atom, its constituent particles, and how electrons occupy specific energy levels within it. Understanding the structure of the atom is crucial as it forms the foundation of the entire field of chemistry.
Structure Of Atom
Discovery of Electron
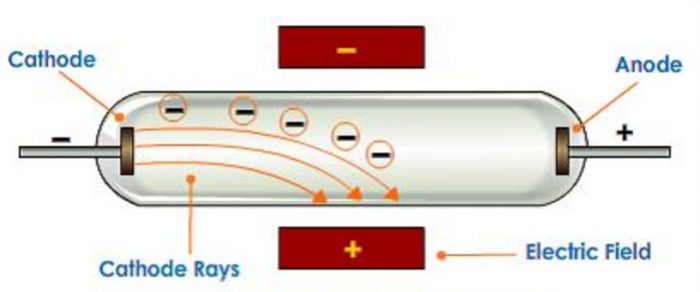
William Crookes in 1879 studied the conduction of electricity through gases at low pressure.
EXPERIMENTAL SETUP
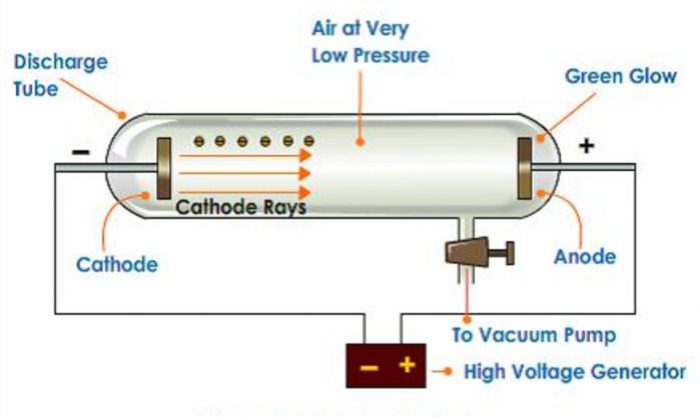
1) He took a discharge tube which is a long glass tube, sealed at both ends and fitted with two metal electrodes.
2) It has a side tube fitted with a stop cock which can be connected to a vacuum pump to reduce the pressure of the gas inside to any desired value.
When a high voltage of about 10000 volts is applied between the electrodes the following results are observed at different pressures :
1) When the gas pressure inside is one atmosphere, no current flows between the electrodes.
2) When the pressure is reduced to about 10 millimetres, the current starts flowing between the electrodes and a coloured glow is observed, the colour depending upon the nature of the gas taken.
3) When the pressure is reduced to about 0.01 millimetre, the glow between the electrodes disappears but the current continues to flow and if a perforated anode is used, a faint greenish glow is observed on the glass walls behind the anode. This shows that some invisible rays are emitted from the cathode which passed through the holes of the anode and strike the wall. These rays were called as cathode rays.
Properties of cathode rays
1)They produce a sharp Shadow of the solid object placed in their path. This shows that cathode rays travel in a straight line.
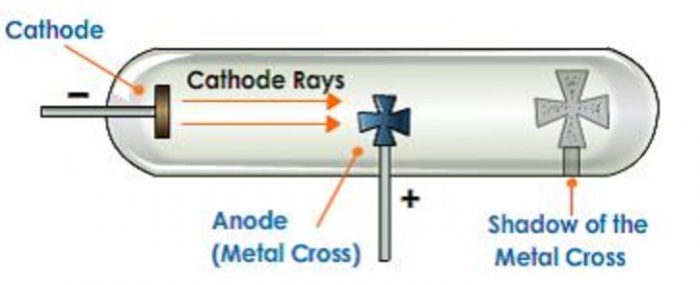
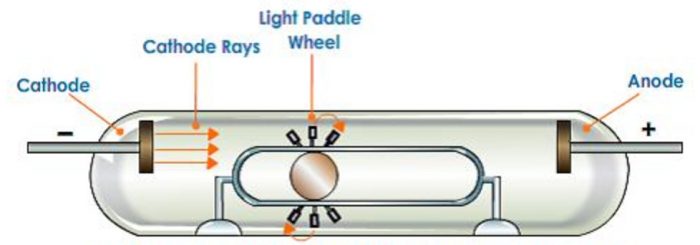
2)If a light paddle wheel mounted on an axle is placed in its path, the wheel begins to rotate. This shows that cathode rays are made up of material particles.
3)When an electric field is applied to the cathode rays, they are deflected towards the positive plate of the electric field. This shows that cathode rays carry a negative charge.
4)When cathode rays strike a metal foil, the latter becomes hot. This indicates that cathode ray produce a heating effect.
5)They produce green fluorescence on the glass walls of the discharge tube as well as on certain other fluorescent substances such as zinc sulphide.
6)They possess penetrating effects ie they can easily pass through thin foils of metals.
The negatively charged material particles constituting the cathode rays are called electrons.
For determination of the ratio of charge/mass of electrons
The experiment was carried out by J.J. Thomson in 1897. He used different discharge tubes fitted with electrodes of different metals. He placed different gases in the tube. He found every time that the ratio of charge/mass of the electron was the same.
The value was found to be 1.76 × 108 coulombs/ g
For the determination of the charge on the electron
The charge on the electron was found by R.A. Millikan in 1917 with the help of his Oil Drop experiment.
The value was found to be 9.11 × 10-10 g
An electron is that fundamental particle which carries 1 unit negative charge and has a mass nearly equal to 1/1837 th of that hydrogen atom.
class 11 chemistry ch 2 notes
Discovery of Proton
Since the atom as a whole is electrically neutral and the presence of negatively charged particles in it was established, therefore, it was thought that some positively charged particles must also be present in the atom.
Goldstein in 1886 performed a discharge tube experiment in which he took a perforated cathode and a gas at low pressure was kept inside the tube, as before.
1)On passing high voltage between the electrodes, it was found that some rays were coming from the side of the anode which passed through the holes in the cathode and produced green fluorescence on the opposite glass wall coated with zinc sulphide. These rays were called anode rays or canal rays or positive rays.
Properties of anode rays
1)They travel in a straight line.
2)They are made up of material particles.
3)They are positively charged.
4)The value of e/m depends upon the nature of the gas taken inside the discharge tube.
5)The value of the charge on the particles constituent of the anode rays is also found to depend upon the nature of the gas taken inside the discharge tube.
6)The mass of the particle constituting the anode rays is also found to be different for different gases taken in the discharge tube.
The charge on these particles is found to be the same as that on the electron i.e. 1.6 × 10-19 coulombs per gram.
The ratio, charge/mass, for each of the particles, is found to be 9.58 × 10-24 g.
These particles were termed protons.
A proton may be defined as a fundamental particle which carries 1 unit positive charge and has a mass nearly equal to that of the hydrogen atom.
Discovery of neutron
Chadwick in 1932, performed some scattering experiments in which he bombarded some light elements like beryllium and boron with fast-moving Alpha particles. He found that some new particles were emitted which carried no charge ie. were neutral but had a mass nearly equal to that of the proton. This particle was termed a neutron.
A neutron may be defined as a fundamental particle which carries no charge but has a mass nearly equal to that of a hydrogen atom or proton.
class 11 chemistry chapter 2 structure of atom notes
Rutherford’s Model of an Atom
Thomson’s model of atom
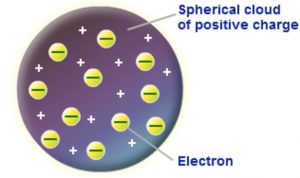
J.J. Thomson in 1904, proposed that an atom was a sphere of positive electricity in which were embedded number of electrons. The stability of the atom was explained as a result of the balance between the repulsive forces between the electrons and their attraction towards the centre of the positive Sphere.This model is compared with a watermelon in which seeds are embedded or with a cake or pudding in which raisins are embedded. That is why this model is called as raisin pudding model a watermelon model.
Limitation
This model could not explain the stability of the atom.
Rutherford Model of atom
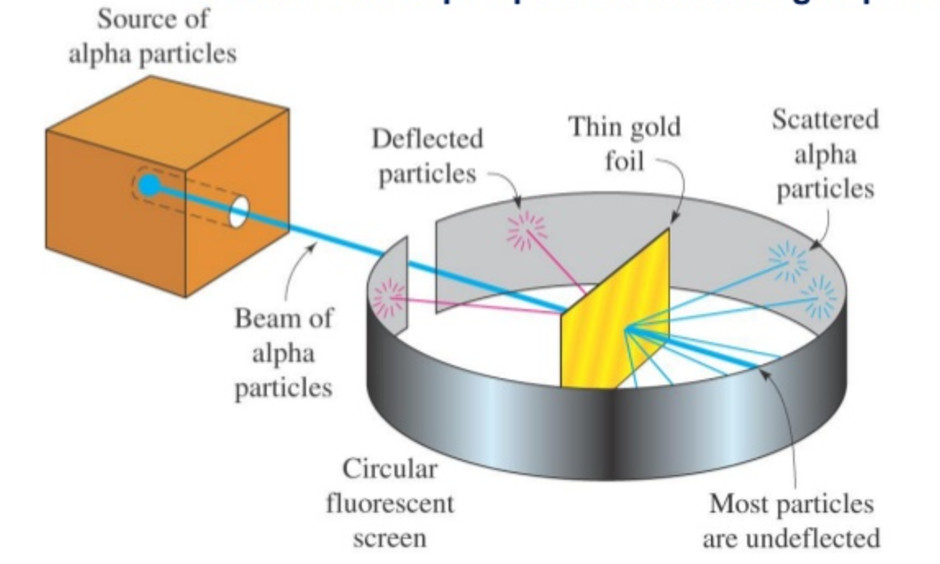
Rutherford in 1911, performed scattering experiment in which he bombarded thin walls of metals like gold, silver, Platinum or copper with a beam of fast moving Alpha particles. The source of Alpha particles was radium, a radioactive substance, placed in a block of lead. Slits were used to get a fine beam. The presence of Alpha particles at any point around the thin foil of gold after striking it was detected with the help of a circular zinc sulphide. The point at which an Alpha particle strikes this screen ,a flash of light is given out.
Observation
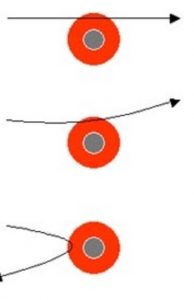
1) Most of the Alpha particles passed through the foil without undergoing any deflection.
2) Few Alpha particles underwent deflection through small angles.
3) Very few were deflected back through an angle greater than 90°
Conclusion
1)Since most of the Alpha particles passed through the foil without undergoing any deflection, there must be sufficient empty space within the atom.
2)Since few alpha particles were deflected through small angles and alpha particles were positively charged particles, these could be deflected only by some positive body present within the atom. The alpha particles deflected were those which passed very close to this positive body.
3)Since some alpha particles were deflected back and alpha particles are heavy particle, these could be deflected back only when they strike some heavier body inside the atom.
4)Since the number of alpha particles deflected back is very very small, this shows that the heavy body present in an atom must be occupying a very very small volume.
The small heavy positively charged body present within the atom was called nucleus.
Rutherford Model of an atom
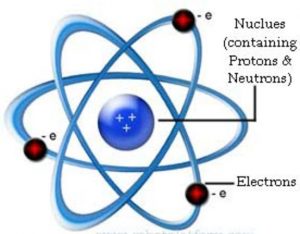
(1) Nucleus is very small in size, carries positive charge and in which the entire mass of the atom is concentrated.
(2) Since electrons have negligible mass ,the mass of the atom is mainly due to protons and neutrons.
(3) Protons and neutrons must be present in the nucleus.
(4) Extranuclear part is the space around the nucleus in which the electrons were distributed.
Drawback of Rutherford’s Model of an atom
(1) Inability to explain the stability of atom
An atom consists of a small, heavy positively charged nucleus in the centre and the electrons were revolving around it. This model was compared with the solar system in which the planets were revolving around the sun and continue to move in their fixed circular paths because the force of attraction was balanced by the centrifugal force.
According to Maxwell’s electromagnetic theory, whenever a charged particle like electron is revolving in a field of force like that of the nucleus, it loses energy continuously in the form of electromagnetic radiation. This is because when a particle is revolving, it undergoes acceleration due to change in direction even if the speed remains constant. Thus, the orbit of the revolving electron will keep on becoming smaller and smaller, following a spiral path and ultimately the electron should fall into the nucleus. The atom should collapse.
Rutherford model could not explain the stability of the atom.
(2) Inability to explain the line spectra of the elements.
(3) Inability to describe distribution of electrons and energies of electrons.
Atomic number
Atomic number of an element = Total number of protons = Total number of electrons
The atomic number is represented by Z
Atomic number is also known as Proton number because the charge on the nucleus depends upon the number of protons.
Mass number is the sum of protons and neutrons.
The mass number is represented by A.
A X Z
Where A is mass number, Z is atomic number and X is the element.
Isotopes
Atoms of the same element having same atomic number but different mass number.
For Example:
(1) Isotopes of Hydrogen
1H1 1H2 1H3
(2) Isotopes of Chlorine
37Cl 35Cl
(3) Isotopes of Carbon
14C 12C 13C
4) Isotopes of Oxygen
16O 17O 18O
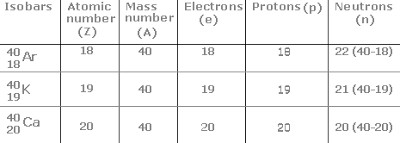
Isobars
Atoms of different elements having different but same mass numbers are called isobars.
For Example:
Isotones
Atoms of different elements which contain the same number of neutrons are called isotones.
For Example:

Isoelectronic
The species containing the same number of electrons are called isoelectronic.

structure of atom class 11 notes ncert
Electromagnetic Wave Theory
Electromagnetic wave theory
This theory was put forward by James clark Maxwell in 1864.
The main points of this theory are:
1)The energy is emitted from any source continuously in the form of radiations and is called the radiant energy.
2)The radiations consist of electric and magnetic fields oscillating perpendicular to each other and both perpendicular to the direction of propagation of the radiation.
3)The radiations possesses wave character and travel with the velocity of light.
The radiations are called electromagnetic radiations or electromagnetic waves.
4)These waves do not require any material medium for propagation.
Characteristics of a wave
1)Wavelength
Wavelength of a wave is defined as the distance between any two consecutive crest or trough.
It is represented by λ (Lambda) and is expressed in A° or m or cm or nm or pm.
1 A° = 10-8 cm = 10-10 m
1nm = 10-9 m, 1pm= 10-12 m
2)Frequency
Frequency of a wave is defined as the number of waves passing through a point in 1 seconds.
It is represented by ν (nu) and is expressed in hertz(Hz) or cycles/second or sec-1
3)Velocity
Velocity of a wave is defined as the linear distance travelled by the wave in 1 seconds.
It is represented by c and is expressed in cm/sec or m/sec.
4)Amplitude
Amplitude of a wave is the height of the crest or the depth of the trough.
It is represented by a and is expressed in the units of length.
5)Wavenumber
Wavenumber is defined as the number of waves present in 1 cm length.
It will be equal to the reciprocal of the wavelength.
It is represented by ![]()
Relation between velocity ,wavelength and frequency of a wave
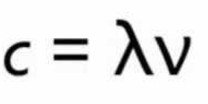
Electromagnetic Spectrum
The different types of electromagnetic radiations differ only in their wavelength and hence frequency.
Their Wave length increases in the following order:
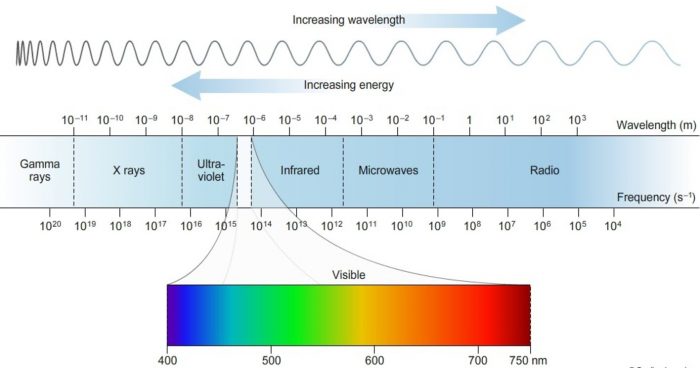
When these electromagnetic radiations are arranged in order of their increasing wavelength or decreasing frequencies, the complete spectrum obtained is called electromagnetic spectrum.
Limitations of electromagnetic wave theory
It could not explain the :
1)The phenomena of black body radiation
2)The photoelectric effect
3)The variation of heat capacity of solid as a function of temperature.
4)The line spectra of atoms with special reference to hydrogen.
Black body radiation
If any substance with high melting point is heated, it first becomes red, then yellow and finally begins to glow with white and then blue light.
If the substance being heated is a black body (which is a perfect absorber and perfect radiator of energy ie. which can emit and absorb all frequencies) the radiation emitted is called black body radiation
The energy of any electromagnetic radiation is proportional to its intensity, ie. square of amplitude and is independent of its frequency or wavelength.
The change of colours shows that on heating,the frequency of the radiations emitted is increasing.
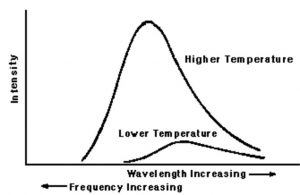
At a given temperature, intensity of radiation emitted increases with decrease of wavelength, reaches a maximum value at a particular wavelength and then start decreasing with further decrease of wavelength.
Photoelectric effect
When radiations with certain minimum frequency ( νo) strike the surface of a metal, the electrons are ejected from the surface of the metal. This phenomenon is called Photoelectric effect
The electrons emitted are called photoelectrons.
1) The electrons are ejected only if the radiation striking the surface of the metal has at least a minimum frequency(νo) . If the frequency is less than νo , no electrons are ejected(hνo). This value is called threshold frequency.
The minimum energy required to eject the electrons is called work function( Wo)
2) The velocity of the electron ejected depends upon the frequency of the incident radiation and is independent of its intensity.
3) The number of photoelectrons ejected is proportional to the intensity of incident radiation.
Planck’s Quantum Theory
Max Planck in 1900, put forward a theory known after his name as Planck’s Quantum theory
The main points of this theory are :
1)The radiant energy is emitted or absorbed not continuously but discontinuously in the form of small discrete packets of energy. Each such packet of energy is called a quantum. In the case of light, the quantum of energy is called Photon.
2)The energy of each quantum is directly proportional to the frequency of the radiation ie.
E=hν
Where h is the proportionality constant, called Planck’s Constant.
Its value is 6.626 × 10 -27 erg sec or 6.626 × 10 -34 joule sec.
3)The total amount of energy emitted or absorbed by a body will be some whole number of quanta.
Hence E= nhν
Explanation of black body radiation
When some solid substance is heated, the atoms of the substance are set into oscillations and emit radiations of frequency, ν. Now ,as heating is continued, more and more energy is being absorbed by the atoms and they emit radiations of higher and higher frequency. As red light has minimum frequency and yellow light has higher frequency, therefore the body on heating becomes first red, then yellow and so on.
Explanation of Photoelectric Effect
1)When light of some particular frequency falls on the surface of metal, the photon gives its entire energy to the electron of the metal atom. The electron will be detached from the metal atom only if the energy of the photon is sufficient to overcome the force of attraction of the electron by the nucleus. That is why photoelectrons are ejected only when the incident light has a certain minimum frequency (threshold frequency ν0)
2If the frequency of the incident light is more than the threshold frequency ,the excess energy is imparted to the electron as kinetic energy.
Greater is the frequency of the incident light, greater is the kinetic energy of emitted electron.
3)When the intensity of light is increased, more electrons are ejected but the energies of these electrons are not altered.
Absorption and Emission Spectra
Electromagnetic spectrum
The electromagnetic spectrum consists of radiation of different wavelength and frequency.
An instrument used to separate the radiations of different wavelength is called spectroscope or spectrograph.
A spectroscope consists of a prism or a diffraction grating for the dispersion of radiation and a photographic film to examine the emergent radiation with the human eye.
The branch of science dealing with the study of spectra is called spectroscopy.
The spectra are broadly classified into:
1) Emission spectra
2) Absorption Spectra
Emission spectra
When the radiations emitted from some source eg: from the sun or by passing electric discharge through a gas at low pressure or by heating some substance to high temperature is passed directly through the prism and then received on the photographic plate, the spectrum obtained is called emission spectrum.
Depending upon the source of radiation, the emission spectra are mainly of two types:
1) Continuous spectrum
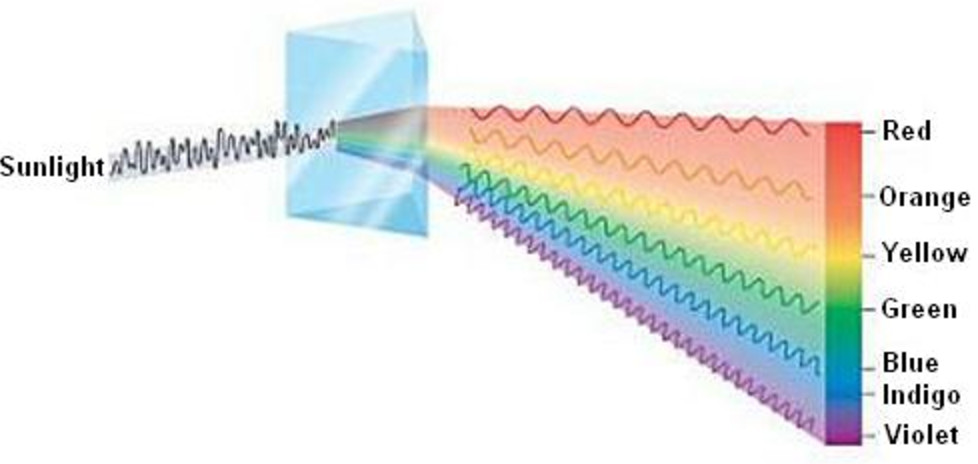
When white light from any source is analysed by passing through a prism, it is observed that it splits up into 7 different wide bands of colour. These colours are so continuous that each of them merges into the next. Hence the spectrum is called a continuous spectrum.
On passing through the prism, the red colour with the longest wavelength deviates the least while the violet colour with the shortest wavelength deviates the most.
2) Line Spectra
When some volatile salt is placed in the bunsen flame or an electric discharge is passed through a gas at low pressure, light is emitted. The colour of light emitted depends upon the nature of the substance.
Sodium emits yellow light while potassium gives out violet light.
If this light is resolved in a spectroscope, some isolated coloured lines are obtained on the photographic plates separated from each other by dark spaces. This spectrum is called the line emission spectrum on line spectrum.
Each line in the spectrum corresponds to a particular wavelength. Sodium always gives two yellow lines.
Absorption spectra
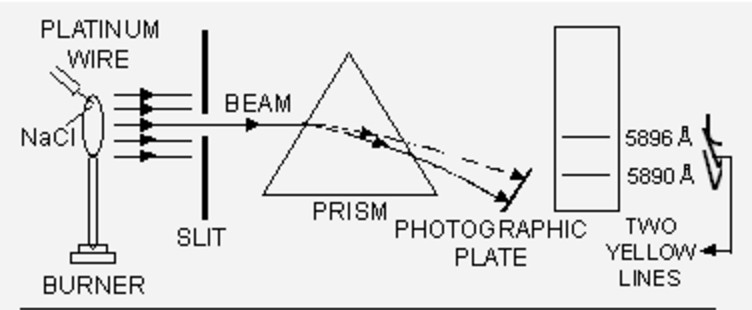
White light from any source is passed through the solution or vapours of a chemical substance and then analysed by the spectroscope, it is observed that some dark lines are obtained. These dark lines are supposed to result from the fact that when the white light is passed through the chemical substance,, the radiations of certain wavelengths are absorbed, depending upon the nature of the element.
The dark lines are at the same place where coloured lines are obtained in the emission spectra for the same substance. The wavelength absorbed were same as were emitted in the emission spectra. The spectrum thus obtained is called absorption spectrum.
Emission spectrum of Hydrogen
When hydrogen gas at low pressure is taken in the discharge tube and the light emitted on passing electric discharge is examined with a spectroscope ,the spectrum obtained is called the emission spectrum of hydrogen.It is found to consist of a large number of lines which are grouped into different series ,named after the discoverer.
Rydberg in 1890 gave a very simple theoretical equation for the calculation of wavelength of these lines.
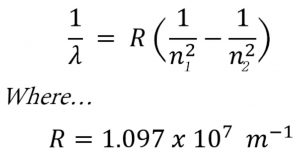
Where R is a constant,called Rydberg constant and has a value of 109677 cm-1 or 1.097 × 107 m-1
n1 and n2 are whole numbers and for a particular series, n1 is constant and n2 varies .
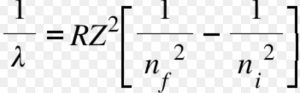
Where Z is the atomic number.
Bohr’s Model of an Atom
Neils Bohr, a Danish physicist in 1913 proposed a new model of the atom. This new model is called Bohr’s Model of Atom.
Postulates of Bohr’s model of an Atom
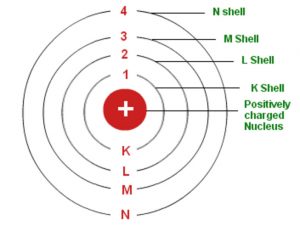
1) An atom consists of a small, heavy positively charged nucleus in the centre and electrons revolve around it in circular orbit.
2) The electrons revolve only in those orbits which have a fixed value of energy. These orbits are called energy levels or stationary States. The energy of the electron revolving in a particular orbit is fixed and does not change with time. The different energy levels are numbered as 1,2,3,4…. or designated as K, L, M, N ,O….. starting from the shells closest to the nucleus.
a) The energies of the different stationary states in case of hydrogen atom are given by the expression
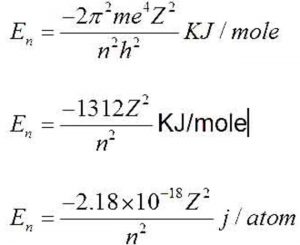
Where Z is the atomic number and n stands for energy level.
The first energy level(n=1) which is closest to the nucleus has lowest energy. The energy of the levels increases as we move outwards starting from the first level.
K< L<M<N<O…..
b) The radii of the stationary states of the hydrogen atom are given by the expression:
rn = a0 n2
Where a0= 5.29 pm is the radius of the first stationary state and is called Bohr’s radius.
For hydrogen like particles ,the radii of the stationary state are given by the expression:
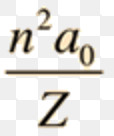
3) Since the electrons revolve only in those orbits which have fixed value of energy ,hence electrons in an atom can have only certain definite or discrete values of energy and not any value of their own. The energy of an electron is quantized.
4)Like energy, the angular momentum of an electron in an atom can have certain definite or discrete values and not any value of its own. The only permissible values of angular momentum are given by the expression:
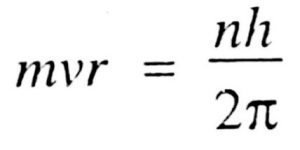
Where m is the mass of the electron, v is the tangential velocity of the revolving electron, r is the radius of the orbit, h is the Planck’s Constant and n is any Integer.
The angular momentum of an electron in an atom is also quantized.
5) When the electrons in an atom are in their lowest energy state, they keep on revolving in the respective orbits without losing energy because energy can neither be lost nor gained continuously. This state of atom is called normal or ground state.
6) Energy is emitted or absorbed only when the electron jumps from one orbit to the other. When energy is supplied to an atom ,an electron in the atom may jump from its normal energy level to some high energy level by absorbing a definite amount of energy. This state of atom is called excited state .Since the lifetime of the electron in the excited state is short, it immediately jumps back to the lower energy level by emitting energy in the form of light of suitable frequency or wavelength.
The amount of energy emitted or absorbed is given by the difference of energies of the two energy levels concerned i.e..
ΔE = E2 – E1
Where E2 and E1 are the energy of the electron in the higher and lower energy levels ΔE is the difference in energies of the two levels.
Energy is always a emitted or absorbed in certain discrete quantities called quantas or photons and not any value.
Electronic Energy as Negative Energy
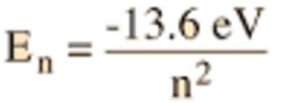
When the electron is at distance Infinity from the nucleus, there is no force of attraction on the electron by the nucleus. Hence, the energy of the electron at distance infinity from the nucleus is taken as zero. As the electron move towards the nucleus, it experiences a force of attraction by the nucleus. As a result some energy is given out. Since its value was already zero, hence now it becomes negative. As the electrons come more and more close to the nucleus, the attraction increases and more energy is released. Hence, the energy of the electron becomes less and less. This explains why the energy decreases as we move from outer to inner shell.
Quantization of Electronic Energy
Quantization means that a quantity cannot change gradually and continuously to have any arbitrary value but changes only abruptly and discontinuously to have certain definite or discrete value. Electron revolve around the nucleus in certain fixed circular orbits in which they can continue revolving without gaining or losing energy. The energy of the electron cannot change continuously but can have only definite values. Thus, we can say that energy of an electron is quantized.
Usefulness of Bohr’s Model
1) It explains the stability of the atom : An electron cannot lose energy as long as it stays in a particular Orbit.
2) It explains the line spectrum of hydrogen : An electron neither emits nor absorbs energy as long as it stays in a particular orbit. When an atom is subjected to electric discharge or high temperature, an electron in the atom may jump from the normal energy level i.e. ground state to some higher energy level ie. excited state. Since the lifetime of the electron in the excited state is short, it returns to some lower energy level or even to the ground state in one or more jumps. During each such jump, energy is emitted in the form of a photon of light of a definite wavelength of frequency.
The frequency of the photon of light the emitted depends upon the energy difference of the two energy level concerned and is given by :
E2 – E1 = hν
Where E2 is the energy of higher energy level and E1 is the energy of lower energy level and h is Planck’s Constant.
Corresponding to the frequency or wavelength of each Photon emitted, there appears a line in the spectrum.
Limitations of Bohr’s model of Atom
1) Inability to explain line spectra of multi-electron atom : When spectroscope with better resolving power were used, it was found that even in case of hydrogen spectrum, each line was split up into a number of closely spaced lines which could not be explained by Bohr’s model of an atom.
2) Inability to explain splitting of lines in the magnetic field (Zeeman effect) and in the electric field (stark effect): If the source emitting the radiation is placed in a magnetic field or in an electric field, it is observed that each spectral line splits up into a number of lines. The splitting the spectral line in magnetic field is called zeeman effect while the splitting of spectral lines in a magnetic electric field is called the stark effect.
3) Inability to explain the three-dimensional model of the atom.
4) Inability to explain the shapes of molecules.
5) Inability to explain de Broglie’s concept of the dual nature of matter and Heisenberg’s Uncertainty Principle.
Hydrogen Spectrum
Hydrogen Spectrum
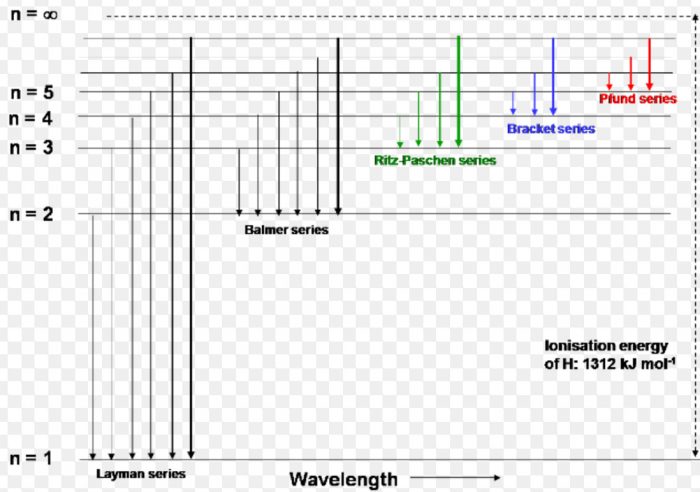
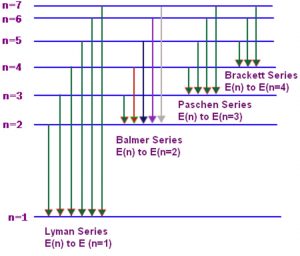
Atomic spectrum of hydrogen consists of a number of lines which have been grouped into 5 series :Lyman, Balmer, Paschen, Brackett and Pfund.
Any given sample of hydrogen gas gas contains a large number of molecules. When such a sample is heated to a high temperature or an electric discharge is passed, the hydrogen molecules splits into hydrogen atoms. The electrons in different hydrogen atoms absorb different amount of energies and are excited to different energy levels. Since the lifetime of electron in these excited states is very small, they return to some lower energy level or even to the ground state in one or more jumps.
Different excited electrons adopt different routes to return to various lower energy levels or the ground state. As a result, they emit different amount of energies and thus produce a large number of lines in the atomic spectrum of hydrogen.
(1) When the electron jumps from energy level higher than n=1 ie. n=2,3,4,5,6 ….to n=1 energy level, the group of lines produced is called lyman series. These lines lie in the ultraviolet region.
(2) The group of lines produced when the electron jumps from 3rd, 4th, 5th or any higher energy level to 2nd energy level, is called Balmer series. These lines lie in the visible region.
(3) Paschen series is obtained by the electronic jump from 4th, 5th or any higher energy level to 3rd energy level.
(4) Brackett series results from electronic transition from 5th, 6th or any higher energy level to 4th energy level.
(5) Pfund series originates by electronic jump from 6th, 7th or any higher energy level to 5th energy level. The spectral line of the last 3 series lies in the infrared region.
Chapter 2 Structure Of Atom Class 11 Notes
Dual Nature of Matter and Radiation
Einstein in 1905 suggested that light has dual nature, i.e. wave nature as well as particle nature.
Matter-wave or a de Broglie wave
Louis de Broglie, a French physicist, in 1924, suggested that all microscopic as well as macroscopic objects possesses dual character. The wave associated with the particle is called a matter wave or a de Broglie wave.
The wavelength of the wave associated with any material particle was calculated as:
In case of photon, if it is assumed to have a wave character, its energy is given by
E = hν
where ν is the frequency of the wave and h is Planck’s Constant.
If the photon is supposed to have particle character, its energy is given by
E = mc2
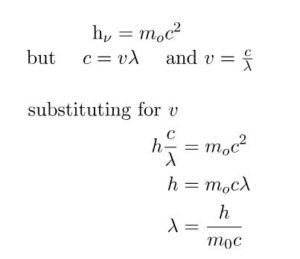
where m is the mass of the photon and c is the velocity of light.
The above equation is called de Broglie Equation and λ is called de Broglie wavelength.
de Broglie wavelength of a particle is inversely proportional to its momentum.
Significance
De Broglie equation relate the particle character with the wave character of matter.
Consider a ball of mass 0.1 kg moving with a speed of 60 m/s.
From de Broglie equation:
λ = h/mv
where h= 6.62 × 10-34
m= 0.1 kg
ν= 60 m/s
we λ = 10-34
Wavelength is too small for ordinary observation.
It has significant only in case of microscopic particles. de Broglie relationship has no significance in everyday life. That is why we do not observe any wave nature associated with the motion of a running car or a cricket ball.
Verification of dual character of electrons
(1) Davisson and Germer’s experiment
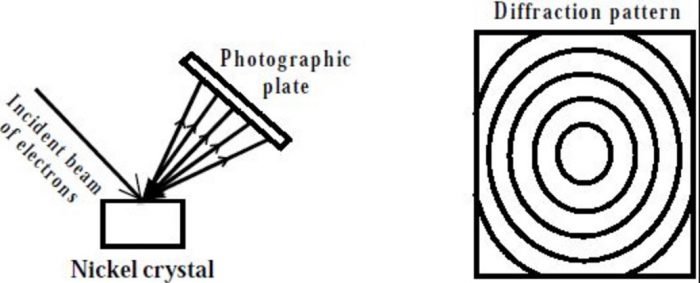
Davisson and Germer in 1927 observed that when a beam of electrons is allowed to fall on the surface of nickel crystal and the scattered or reflected rays are received on a photographic plate, a diffraction pattern (consisting of number of concentric rings) similar to that produced by X-ray, is obtained. Since X-ray are electromagnetic waves i.e. they are confirmed to have wave character, therefore electrons must also have wave character.
(2) Thomson’s experiment
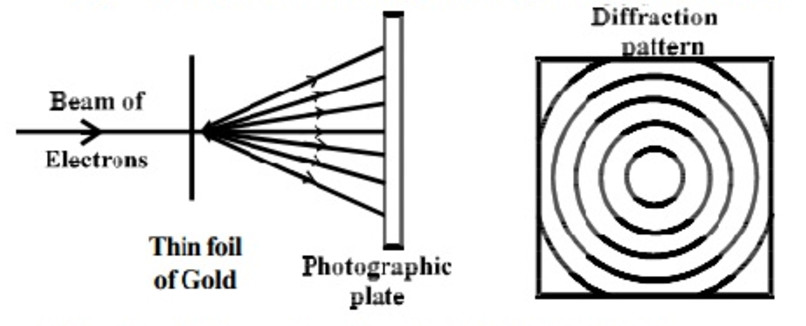
He performed experiment with thin foil of gold in place of nickel crystal. He observed that if the beam of electrons after passing through the thin foil of gold is received on the photographic plate placed perpendicular to the direction of the beam, a diffraction pattern is observed.
Verification of Particle Character
When an electron strikes a zinc sulphide screen, a spot of light known as scintillation is produced. Since a scintillation is localised on the zinc sulphide screen, therefore, the striking electron which produces it also must be localised and not spread out on the screen. The localised character is possessed by the particles .Hence electrons has particle character.
(2) Milliken Oil Drop experiment for determination of charge on electron also show that electron has particle character.
(3) The phenomenon of black body radiation and Photoelectric effect also Prove the particle nature of radiation.
De Broglie wavelength of the electron from the potential applied to accelerate it
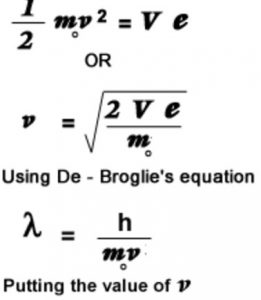
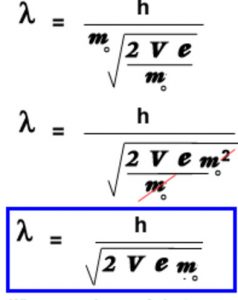
Derivation of Bohr’s postulate of angular momentum from de Broglie equation
According to Bohr’s model, the electron revolves around the nucleus in circular orbits.
According to de Broglie concept, the electron is not only a particle but has a wave character.
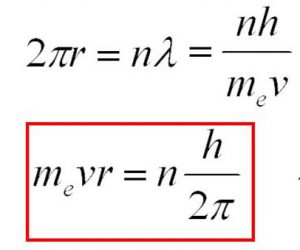
Heisenberg’s Uncertainty Principle
Werner Heisenberg, a German physicist, in 1927 gave a principle about the uncertainty in simultaneous measurement of position and momentum of small particles.
Heisenberg’s uncertainty Principle states that:
It is impossible to measure simultaneously the position and momentum of a small particle with absolute accuracy or certainty. The product of the uncertainty in the position ( Δx ) and the uncertainty in the momentum(Δp) is always constant and is equal to or greater than h/4π, where h is the Planck’s Constant ie.
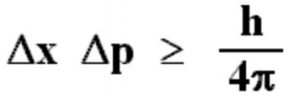
Significance of Heisenberg Uncertainty Principle
Heisenberg’s uncertainty Principle holds good for all objects but it is significant only for microscopic particles. The energy of the photon is insufficient to change the position and velocity of bigger bodies when it collides with them.
For a particle of mass 1 mg, we have
Δx . Δv = h / 4π m
Δx . Δv = 6.626 × 10-34 / 4 × 3.1416 × ( 106 )
Δx . Δv = 10-28 m2 s-1
The product Δx and Δv is extremely small. For particle of mass greater than 1 mg, the product will be still smaller. Hence these values are negligible.
For a microscopic particle like an electron, we have
Δx . Δv = 6.626 × 10-34 / 4 × 3.1416 × ( 9.11 × 10-31)
Δx . Δv = 10-4 m2 s-1
If uncertainty in position is 10-4 ,uncertainty in velocity will be 0.1 m/s.
Bohr’s concept of fixed circular path with definite position and momentum of electron have been replaced by stating that the electron has the probability of having a given position and momentum.
Electron cannot exist in the nucleus
This is because the diameter of the atomic nucleus is of the order 10-15 m. If the electron were to exist within the nucleus, the maximum uncertainty in its position would have been 10-15 m .Taking the mass of electron as 9.1 × 10 -31kg, the minimum uncertainty in velocity can be calculated by applying uncertainty Principle as follow:
Δ x . Δ p = h/4π
Δ x. (m × Δv) = h/4π
Δ v = h/ 4π × Δx × m
Δ v = 6.626 × 10-34 / 4 × 3.1416 × 10-15 × 9.1 × 10 -31
Δ v = 5.77 × 1010 m/s
This value is much higher than the velocity of light ( 3 × 108 )and hence is not possible.
Quantum Mechanical Model of an Atom
Quantum mechanics, as developed by Erwin Schrodinger in 1926, is based on the wave motion associated with the particles. For the wave motion of the electron in the three dimensional space around the nucleus, he put forward an equation known as Schrondinger wave equation.
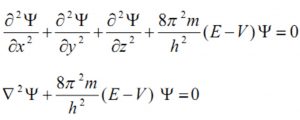
where ψ is the amplitude of the wave where the coordinates of the electrons are ( x,y,z) ,E is the total energy of the electron, V is its potential energy, m is the mass of the electron and h is the Planck’s Constant,
![]() represents second derivative of Ψ w.r.t. x.
represents second derivative of Ψ w.r.t. x.
In short Schrodinger wave equation is written as
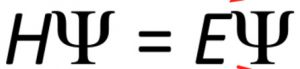
Where H is a mathematical operator ,called Hamiltonian operator.
The solution of Schrodinger wave equation for an electron in an atom gives the value of E and Ψ .The value of E represents the quantised value of energy which the electron in the atom can have. The corresponding value of ψ are called wave functions.
Significance of the wave function
In case of light or sound, the square of the amplitude of the wave at any point gives the intensity of the sound or light at that point, Similarly the square of the amplitude of the electron wave ie ψ2 at any point gives the intensity of the electron wave at that point which in view of Heisenberg Uncertainty Principle means probability of finding the electron at that point.
ψ2 at any point gives the probability of finding the electron at that point i.e. electron density at that point.
The region around the nucleus which represents the electron density at different points is called an orbital.
The wave function for an electron in an atom is called orbital wave function or atomic orbital. Since an electron can have any wave function ,therefore, there are many atomic orbitals in an atom.
Probability provides the best possible description of a situation which cannot be described with certainty.
The electron spends most of its time in the volume of Sphere bounded by that distance and for rest of the time, the electron can be found outside the volume of the sphere.
The region of space around the nucleus which describes the probability of finding an electron of given energy in terms of dots is called an electron cloud.
An atomic orbital may be defined as the three dimensional space around the nucleus within which the probability of finding an electron of given energy is maximum.
Features of quantum mechanical model of atom
1)The electrons in an atom have only quantized value of energy.
2)These quantized values of energy are obtained from the solution of Schrodinger Wave Equation. The values of the wave function Ψ are also obtained from the solution of Schrodinger Wave Equation.
3)The wave function Ψ is simply a function of coordinates of the electron and has no physical significance as such.ψ2 give the probability of finding the electron at that point i.e. electron density at that point.
4) By finding ψ2 at different points around the nucleus in an atom, we can predict the region of space around the nucleus within which the probability of finding the electron with a definite value of energy is maximum. This space around the nucleus is called orbital .Wave function Ψ is called orbital wave function or atomic orbital.
5) Since an electron can have many wave function therefore there are many atomic orbitals in an atom.
6)The orbital wave function contains all the information about an electron in an atom and the quantum mechanics helps to extract this information.
Application of Schrodinger Wave Equation to hydrogen atom
When Schrodinger Wave Equation is solved for hydrogen atom, the solution gives possible values of the energy ( E ) .
These values of energy correspond to the energy levels which the electron in an atom can occupy.
These values of energy are called eigen values.
The corresponding values of wave functions ( Ψ ) are called eigenfunctions.
Quantum Numbers
An atom contains a large number of orbitals. These are distinguished from each other on the basis of their shape, size and orientation in space. These characteristics of an orbital are expressed in terms of three numbers, called principal, azimuthal and magnetic quantum number.
Quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. location, energy, the type of Orbital occupied, space and orientation of that orbital.
Principal Quantum Number
It tells the principal energy level or shell to which the electron belongs.
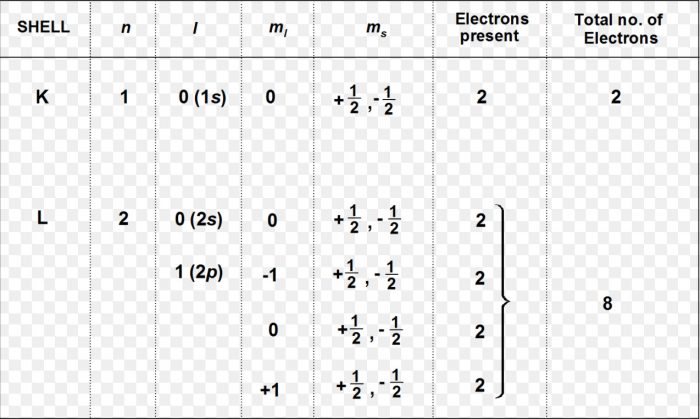
It is donated by the letter n and can have any integral value except 0 i.e. n=1,2,3,4…. etc.
The various principal energy shells are also designated by the letters K,L,M,N,O….starting from the nucleus.
This number helps to explain the main lines of the spectrum on the basis of the electronic jump between these shell.
(a) It gives the average distance of the electron from the nucleus, i.e. it largely determined the size of the electron cloud.
(b) It completely determine the energy of the electron in hydrogen atom and hydrogen like particles.
For the first principal shell( K),n=1 which means that this energy shell is lowest energy and lies closest to the nucleus.
For the second principal shells( L),n=2 and for the third principal shell (M), n=3 and so on.
The energies of the various principal shells follow the sequence:
K<L<M<N<O…..
1<2<3<4<5……
The maximum number of electrons present in any principle shell is given by 2n2 Where n is the number of principal shell.
Azimuthal or Angular momentum Quantum Number
Within the same principal Shell, there are present a number of sub shells or sub levels of energy. As a result, the number of electronic jumps increases and so is the number of lines.
Thus, this number helps to explain the fine lines of the spectrum.
Azimuthal quantum number tells about the :
(1) Number of sub shells present in the main shells.
(2) The angular momentum of the electron present in any sub shell.
(3) The relative energies of the various sub shells.
(4) The shapes of the various sub shells present within the same principal Shell.
For a given value of n, it can have any integral value ranging from 0 to n-1.
For 1st Shell (K), n=1, l can have only one value i.e., l=0
For the 2nd Shell (L), n=2, l can have two values i.e. l=0 and 1
For the 3rd Shell ( M), n= 3, l can have three values i.e. l=0, 1, 2
For the 4th shells ( N ), n=4, l can have 4 values i.e. l=0, 1, 2, 3
Depending upon the values of l, i.e. l = 0, 1, 2 and 3,the different sub shells are designated as s,p,d and f .These notations are the initial letters of the words, sharp, principal, diffused and fundamental formerly used to describe different spectral lines.
l = 4 is called g sub shell, l = 5 is called is h sub shell.
(1) First principal shell (K shell or n=1 ) has only one sub shell called the s sub shell.
(2) Second principal shell (L shell or n=2) has only 2 sub shells i.e. s sub shell ( l = 0 ) and p sub shell (l=1)
(3) Third principal shell (M shell or n= 3) has three sub shells i.e. s sub shell ( l=0 ), p sub shell (l= 1) and d sub shell (l=2 )
(4) Fourth principal shell (N shell or n=4 ) has four sub shell i.e. s sub shell ( l=0), p sub shell ( l= 1), d sub shell ( l=2) and f sub shell ( l=3).
The number of sub shells present in any principal shell is equal to the number of principal Shell or the principal quantum number.
The energies of different sub shells present within the same principal are found to be in order
s < p< d < f
i.e. an electron in the s – sub shell has lower energy than that in the p sub shell of the same principal Shell.
The maximum number of electrons in the s, p, d and f sub shell are 2, 6,10 and 14.
Magnetic Quantum Number
This quantum number is required to explain the fact that when the source producing the line spectrum is placed in a magnetic field, each spectral line splits up into a number of lines.
An electron due to its orbital motion around the nucleus generates an electric field. This electric field in turn produces a magnetic field which can interact with the external magnetic field. Thus under the influence of external magnetic field, the electrons of a sub shell can orient themselves in certain preferred regions of space around the nucleus called orbitals.
The magnetic quantum number determines the number of orbitals present in any sub shell.
The magnetic quantum number determines the number of preferred orientation of the electron present in a sub shell.
The magnetic quantum number is denoted by the letter m or ml and for a given value of l, it can have all the values ranging from -l to + l including zero.
For every value of l, m has 2l + 1 values.
(1) For l=0, m can have only one value. This means that s sub shell has only one orientation in space. s sub shell has only one orbital called s – orbital.
(2) For l=1( p sub shell), m can have three values i.e. m = -1,0, +1.p sub shell has 3 orbitals. Since these 3 orbitals are oriented along x axis, y axis and z axis, therefore they are commonly referred to as px, py and pz.
(3) For l=2( d sub shell), m can have five values i.e. m =-2, -1,0, +1, +2 .d sub shell has 5 orbitals.
(4) For l=3 (f sub shell ), m can have 7 values i.e. m = -3, – 2, -1,0, +1, +2, +3 there are 7 different orientation of f sub shells. f sub shell has 7 orbitals.
All the three p orbitals of a particular principal shell have the same energy in the absence of a magnetic field. All the five d orbitals of a particular shell have the same energy and all the 7 f orbitals have same energy.
These orbitals of the same sub shell having equal energy are called degenerate orbitals.
In the presence of an external magnetic field, this degeneracy is broken and orbitals of the same sub shell acquire slightly different energy. This cause the splitting of a given spectral line into many.
Spin Quantum Number
The electron in an atom not only moves around the nucleus but also spin about its own axis. This number give the information about the direction of spinning of the electron present in any orbital.
It is represented by s or ms.
Since the electron in an orbital can spin either in clockwise direction or in the anticlockwise direction, hence for a given value of m, s can have only two values i.e. + ½ and – ½ or these are very often represented by two arrows pointing in the opposite direction i.e. ↑ or ↓.
This quantum number helps to explain the magnetic properties of the substance. A spinning electron behaves like a micromagnet with a definite magnetic moment. If an orbital contains 2 electrons, the two magnetic moment opposes and cancel each other.
In an atom, if all the orbitals are fully filled, net magnetic moment is zero and the substance is diamagnetic.
If some half filled orbitals are present, the substance has a net magnetic moment and is paramagnetic.
Pauli Exclusion Principle
Wolfgang Pauli a German physicist in 1925 put forward a principle known after his name as Pauli exclusion principle.
No two electrons in an atom can have the same set of four quantum number.
In an atom, any two electrons may have the same values for any of the three quantum numbers but the 4th must be different.
Any particular orbital is described by three quantum numbers i.e. n, l and m.
For Ex: 3s orbital has n=3, l=0 and m=0.
Since for each value of m, there are two values of the spin quantum number i.e. + ½ and – ½ therefore 3s orbital can have two electrons, one with quantum number n=3 , l=0, m=0 and s= + ½ and other with quantum number, n=3 , l=0, m=0 and s= – ½ .
An orbital can have a maximum two electrons and these have opposite Spins.
Number of sub shells in nth shell = n.
Number of orbitals in a sub shell = 2l + 1
Maximum number of electrons in a sub shell=2(2l +1)
Number of orbitals in nth shells = n2
Maximum number of electrons in nth shell = 2n2
Shapes of Atomic Orbital
An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum.
The probability at any point around the nucleus is calculated using schrodinger wave equation and is represented by the density of the points.
Shape of s orbital
For the coordinates( x, y, z) of the electron with respect to the nucleus, schrodinger Wave equation can be solved to get the values of the orbital wave function ψ. But Ψ has no physical significance. The square ψ2 has the significance as it gives the electron probability density of the electron at that point.
1) The probability of 1s electron is found to be maximum near the nucleus and decreases as the distance from the nucleus increases.
2) In case of 2s electrons, the probability is again maximum near the nucleus and then decreases to zero and increases again and then decreases as a distance from the nucleus increases.
3) The intermediate region where probability is zero is called nodal surface or node.
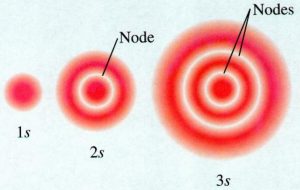
2s orbital differ from 1s orbital in having node within it.3s has two nodes.Any ns orbital has ( n-1) nodes.
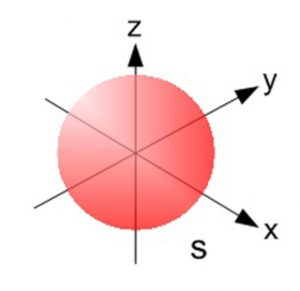
1) The probability of finding the electron belonging to a s orbital of any main shell is found to be identical in all directions at a given distance from the nucleus. Hence s – orbital is spherical in shape which is symmetrical around the nucleus.
2) For s orbital azimuthal quantum number l = 0. Magnetic quantum number m is also equal to 0.s orbital has only one orientation. The only shape having one orientation is a sphere. s orbital is spherical in shape.
1s, 2s, 3s etc all have spherical shape, they differ in:
1) the number of nodes
2) size and energy. These increases with increase in principal quantum number, n.
Shapes of P orbital
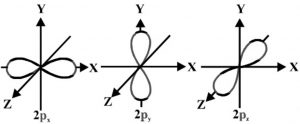
It is found that the probability of finding the electron is maximum in two lobes on the opposite side of the nucleus. This gives rise to dumb-bell shape for the p orbital.
The probability of finding a particular P electron is equal in both the lobes. There is a plane passing through the nucleus on which the probability of finding the electron is almost zero. This is called a nodal plane.
For p orbital l= 1, m = -1, 0, +1 .Thus p orbital has three different orientation designated as px, py, pz depending upon whether the electron density is maximum along the x-axis, y axis and Z axis.
P orbital have directional characteristics and hence are helpful in predicting the shape of molecules.
As n increases these p orbitals become larger in size and have higher energies. The three p orbitals belonging to a particular energy shells have equal energies and are called degenerate.
Shapes of d orbital
For d orbital, l=2.Hence m= -2, -1, 0, +1, +2
There are 5 d orbitals, depending upon the axes along which or between which their electron clouds are concentrated, their names and shapes are:
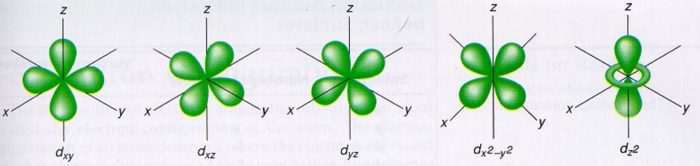
d z2 has a doughnut shaped electron cloud in the centre whereas others clover leaf shape.
Number of nodes in any orbital= (n – l -1)
Energy Level Diagram
Energies of orbitals of hydrogen and hydrogen like particles depend upon the value of principal quantum (n) number only , those of multi-electron atoms depend both upon principal quantum number ( n ) as well as azimuthal quantum number(l).
Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams.
Important observations from energy level diagrams of multi electron atoms are:
1) The sub shell of a particular shell do not have equal energies. For Ex: 2s and 2p have different energies.
2) In a particular shell, sub shell with lower value of l has lower energy. In the second shell, 2s ( l=0) has lower energy than 2p ( l=1) .In the 3rd shell, energies are in the order: 3s < 3p < 3d.
In the 4th shells, they are in the order of 4s <4p< 4d< 4f.
3) For the same value of n, the differences between energies of s and p sub shell is small whereas between p and d sub shell, it is large and so on.
4) As the value of n increases ,the sub shell of lower shell may have higher energy than that of a higher shell, 3d has higher energy than 4s.
Difference of energy in d sub shell of multi electron atoms
In hydrogen atom, the only force of interaction is the force of attraction between the negatively charged electron and the positively charged nucleus.
But in a multi electron atom, in addition to the force of attraction between the electrons and the nucleus, there are forces of repulsion among the electrons.
The atom is stable because the net forces of attraction are greater than forces of repulsion. The repulsive forces are on the electrons of the outer shell by the electrons of the inner shell.
The attractive forces on the electron increases with increase of the nuclear charge. But these attractive forces on the outer shell electrons are greatly reduced by the presence of inner shell electrons which produce a screening effect or shielding effect between the outer shell electrons and the nucleus.
The net positive charge experienced by the electrons is does much less. This is known as effective nuclear charge.
Effective nuclear charge experienced by an electron depends upon the shell and the shape of the orbital in which that electron is present.
1) s orbital ,being spherical in shape, shields the electrons from the nucleus more effectively then p orbital which in turn shields more effectively then d orbital.
2) Within the same shell, s – orbital are more attracted to the nucleus and hence have lower energy than d orbital which in turn are more attracted to the nucleus and hence have lower energy than the d orbital.
The energies of the orbitals depend both on the values of n and l.
3) As effective nuclear charge depends upon the actual nuclear charge, similar orbitals of different atoms do not have the same energy. These decrease with increase in nuclear charge.
Filling of orbitals in atoms
The filling of electrons into the orbitals of different atom take place according to 3 rules:
1)Aufbau principle
The principle states that:
In the ground state of the atoms ,the orbitals are filled in order of their increasing energies. Electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only when the lower energy orbitals are filled.
The order in which the energies of the orbital increases and hence the order in which the orbitals are filled is as follow:
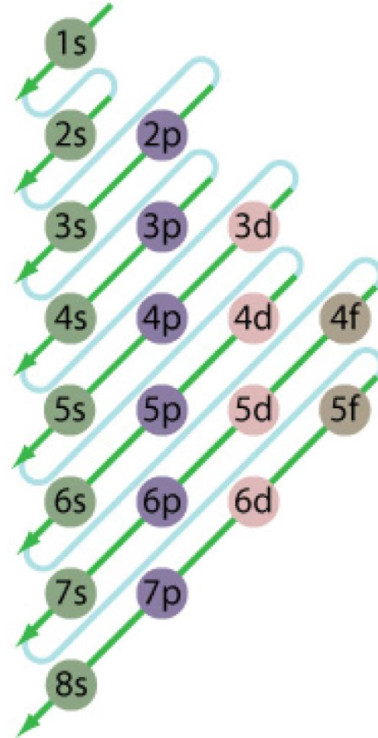
The energy of an orbital depends upon the sum of the values of principal quantum number( n ) and azimuthal quantum number. This is called (n + l) rule.
In neutral isolated atom ,the lower the value of ( n + l) for an orbital, lower is its energy. However, if the two different types of orbital have the same value of ( n+ l) ,the orbital with lower value of n has lower energy.
Pauli Exclusion Principle
An orbital can have maximum 2 electrons and these must have opposite spins.
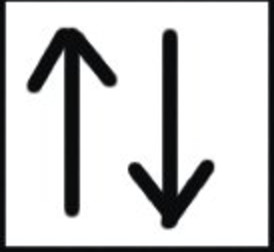
The two electrons have opposite spins i.e. if one is revolving clockwise, the other is revolving anticlockwise.
Hund’s rule of maximum multiplicity
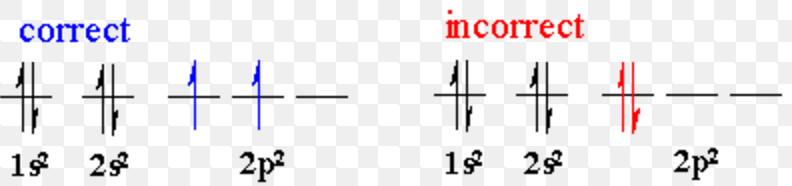
It deals with the filling of electrons into degenerate orbitals of the same sub shell ( p, d and f)
Electron pairing in p, d and f orbital cannot occur until each orbital of a given sub shell contains one electron or is singly occupied.
Electrons being identical in charge, repel each other when present in the same orbital. This repulsion can be minimised if two electrons move as far apart as possible by occupying different degenerate orbitals.
All the singly occupied orbitals will have parallel spin i.e. in the same direction either clockwise or anticlockwise. This is due to the fact that two electrons with parallel spin will encounter less into electronic repulsion in space than when they have opposite spins.
Electronic Configuration of Elements
The distribution of electrons into different shells, sub shells and orbitals of an atom is called its electronic configuration.
The electronic configuration of any orbital can be represented as: nlx
n is the number of principal shell, l = symbol of the sub shell or orbital, x= number of electrons present in the orbital
4p1 means that p- sub shell of the 4th main shell contain one electron.
In certain elements when the two sub shells differ slightly in their energies, an electron may shift from a sub shell of lower energy to a sub shell of higher energy only if such a shift results in the symmetrical distribution of the electrons in the various orbitals of the sub shell of higher energy.
Symmetrical distribution: The electronic configuration in which all the orbitals of the same sub shell are either completely filled or are exactly half filled are more stable because of symmetrical distribution of electrons.
1) The expected electronic configuration of chromium
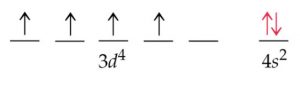
If one of the 4s electron shifts to the vacant 3d orbital ,the distribution of the electron will become more symmetrical and this will impart extra stability.
The actual electronic configuration of chromium
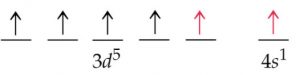
2) The expected electronic configuration of copper
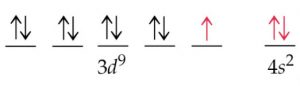
If one of the 4s electron shifts to the vacant 3d orbital ,the distribution of the electron will become more symmetrical and this will impart extra stability.
The actual electronic configuration of copper
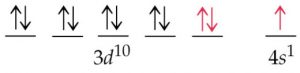
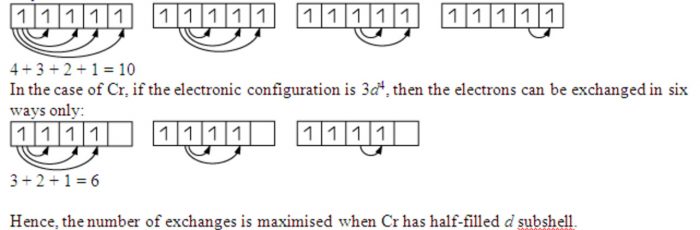
Exchange energy
The electrons with parallel spins present in the degenerate orbitals tend to exchange their position .The energy released during this exchange is called exchange energy.
The number of exchanges that can take place is maximum when degenerate orbitals are exactly half filled or completely filled.As a result, the exchange is maximum and so is the stability.