Acid Bases and Salts Class 10 Notes Science Chapter 2
CBSE Class 10 Science Notes CHAPTER 2 Acids Bases and Salts
Acid – A substance that produces hydrogen ions (H+) in aqueous solutions.
For Example – Sulphuric Acid (H2SO4), Hydrochloric Acid (HCl).
General properties of acids:
- They have a sour taste.
- They turn blue litmus to red.
- They conduct electricity in solution form.
- They release H+ ions in aqueous solution.
Dilute Acid : A dilute acid contains a small amount of acid (lower concentration of hydroniumions) and a large amount of water.
| Vinegar | Acetic Acid |
| Orange | Citric Acid |
| Lemon | Citric Acid |
| Tamarind | Tartaric Acid |
| Tomato | Oxalic Acid |
| Sour milk (Curd) | Lactic Acid |
| Ant and Nettle sting | Methanoic Acid |
(i) Reaction of Acid with Metal
Na (metal) + H2 SO4 (acid) → H2 (hydrogen gas) + Na SO4 (salt)
(ii) Reaction of Acid with Carbonates
Na2 CO3 (s) + 2 HCl (aq) → 2NaCl (aq) + H2O(l) + CO2(g)
(iii) Reaction of Acid with Bicarbonates
NaHCO3 (s) + HCl (aq) → NaCl(aq) + H2O (l) + CO2 (g)
Base – A substance that produces hydroxide ions (OH–) in aqueous solutions.
For Example – Sodium Hydroxide (NaOH), Potassium hydroxide (KOH)
General properties of bases:
- They have a bitter taste.
- They are soapy to touch.
- They turn red litmus to blue.
- They conduct electricity in solution form.
- They release OH– ions in aqueous solution
Reactions of Bases
(i) Reaction with Metals
2NaOH + Zn → Na2ZnO2 + H2
(ii) Reaction with Non-metallic Oxides
2NaOH + CO2 → Na2CO3 + H2O
(iii) Reaction with Acids
NaOH + HCl → NaCl + H2O
- Alkalis
All bases do not dissolve in water. An alkali is a base that dissolves in water. Common alkalis are
- NaOH Sodium hydroxide
- KOH Potassium hydroxide
- Calcium hydroxide
- Ammonium hydroxide
Note : All alkalis are bases but all bases are not alkalis.
Neutralisation Reaction: An acid neutralizes a base when they react with each other and respective salt and water are formed.
Acid + Base → Salt + Water
Since, the reaction between acid and base both neutralize each other, hence, it is also known as Neutralization Reaction.
Examples: Sodium chloride and water are formed when hydrochloric acid reacts with sodium hydroxide (a strong base).
In a similar way, calcium chloride is formed along with water when hydrochloric acid reacts with calcium hydroxide (a base).

|
Strong Acids |
Strong Base |
|
Weak Acids |
Weak Base A base which does not completely dissociate into its ions in aqueous solution. For example: Ammonium hydroxide (NH4OH). |
Differences Between Acid And Base ;
| Acids | Bases |
| Sour in taste Derived from Greek word’ ACIDUS’ |
Bitter in taste |
| Changes blue litmus into red | Changes red litmus into blue |
| e.g. Hydrochloric acid HCl | e.g. Sodium hydroxide NaOH |
Sulphuric acid  |
Potassium hydroxide KOH |
Nitric acid |
Calcium hydroxide |
Acetic acid |
Ammonium hydroxide |
- Acid – Base Indicator : Substances which indicate the presence of an acid or base in a solution.
- Litmus solution – It is a natural indicator. It is a purple dye extracted from Lichens. Other examples are Red Cabbage and coloured petals of Petunia and turmeric.
- Onion: Paste or juice of onion loses its smell when added with base. It does not change its smell with acid.
Vanilla: The smell of vanilla vanishes with base, but its smell does not vanish with an acid. - Olfactory indicators : Show odour changes in acidic or basic media. E.g. onion and clove .
| Indicator | Original Colour | Acid | Base |
| Red litmus | Red | No Change | Blue |
| Blue litmus | Blue | Red | No change |
| Turmeric | Yellow | No Change | Reddish brown |
| Red cabbage juice | Purple | Reddish | Greenish yellow |
| Phenolphthalein | Colourless | Colourless | Pink |
| Methyl Orange | Orange | Red | Yellow |
| Onion | n/a | No change | Smell vanishes |
| Vanilla | n/a | No change | Smell vanishes |
Concept of pH scale
Strength of an acid or base can be determined using a pH scale. It is a scale to measure the hydrogen ion concentration in a solution. The p stands for ‘potenz’, it is a German word which means power.

For water or neutral solutions : pH = 7
For acidic solutions : pH < 7
For basic solution : pH > 7
Importance of pH in everyday life
(i) pH in our digestive system: Our stomach produces hydrochloric acid that helps in the digestion of food. During indigestion the stomach produces too much acid and this causes pain and irritation. To get rid of this pain, antacids like magnesium hydroxide [Mg(OH)2] also known as milk of magnesia and sodium hydrogen carbonate (baking soda) are used to neutralize excess acid.
(ii) Tooth decay caused by acids: Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating. When the pH of acid formed in the mouth falls below 5.5, tooth-decaying starts. The best way to prevent this is to clean the mouth after eating food. Using toothpastes, which are generally basic, for cleaning the teeth can neutralise the excess acid and
prevent tooth decay.
(iii) pH of soil and plant growth: Most of the plants require a specific pH range (close to 7) for their healthy growth. If the soil is too acidic or basic, the plants grow badly or do not grow at all. pH of the soil can be adjusted by using certain chemicals. For example, if the soil is too acidic then it is treated with materials like quicklime or slaked lime. On the other hand, if the soil is too alkaline then alkalinity can be reduced by adding decaying organic matter.
(iv) pH of Acid Rain : When pH of rain water is less than 5.6 it is called acid rain. Flow of acidic rain in water bodies makes them acidic causing a threat to the survival of aquatic life. It also results in damage of structures made with marble like Taj Mahal.
Salts: Salts are the ionic compounds which are produced after the neutralization reaction between acid and base. Salts are electrically neutral. There are number of salts but sodium chloride is the most common among them. Sodium chloride is also known as table salt or common salt. Sodium chloride is used to enhance the taste of food.
Characteristics of salt:
- Most of the salts are crystalline solid.
- Salts may be transparent or opaque.
- Most of the salts are soluble in water.
- Solution of the salts conducts electricity in their molten state also.
- The salt may be salty, sour, sweet, bitter and umami (savoury).
- Neutral salts are odourless.
- Salts can be colourless or coloured.
Family of Salt: Salts having common acidic or basic radicals are said to belong to the same family.
Example:
(i) Sodium chloride (NaCl) and Calcium chloride (CaCl2) belongs to chloride family.
(ii) Calcium chloride (CaCl2) and Calcium sulphate (CaSO4) belongs to calcium family.
(iii) Zinc chloride (ZnCl2) and Zinc sulphate (ZnSO4) belongs to the zinc family.
Neutral, Acidic and Basic Salts:
(i) Neutral Salt: Salts produced because of reaction between a strong acid and strong base are neutral in nature. The pH value of such salts is equal to 7, i.e. neutral.
Example : Sodium chloride, Sodium sulphate. Potassium chloride, etc.
Sodium chloride (NaCl): It is formed after the reaction between hydrochloric acid (a strong acid) and sodium hydroxide (a strong base).
Sodium Sulphate (Na2SO4): It is formed after the reaction between sodium hydroxide (a strong base) and sulphuric acid ( a strong acid).
Potassium Chloride (KCl): It is formed after the reaction between potassium hydroxide (a strong base) and hydrochloric acid (a strong acid).
(ii) Acidic Salts: Salts which are formed after the reaction between a strong acid and weak base are called Acidic salts. The pH value of acidic salt is lower than 7. For example Ammonium sulphate, Ammonium chloride, etc.
Ammonium chloride is formed after reaction between hydrochloric acid (a strong acid) and ammonium hydroxide (a weak base).
Ammonium sulphate is formed after reaction between ammonium hydroxide (a weak base) and sulphuric acid (a strong acid).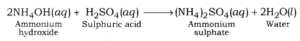
(iii) Basic Salts: Salts which are formed after the reaction between a weak acid and strong base are called Basic Salts. For example; Sodium carbonate, Sodium acetate, etc.
Sodium carbonate is formed after the reaction between sodium hydroxide (a strong base) and carbonic acid (a weak acid).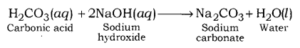
Sodium acetate is formed after the reaction between a strong base, sodium hydroxide (a strong base) and acetic acid, (a weak acid).
Cause of formation of acidic, basic and neutral salts:
- When a strong acid reacts with a weak base, the base is unable to fully neutralize the acid. Due to this, an acidic salt is formed.
- When a strong base reacts with a weak acid, the acid is unable to fully neutralize the base. Due to this, a basic salt is formed.
- When equally strong acid and a base react, they fully neutralize each other. Due to this, a neutral salt is formed.
pH value of salt:
- Neutral salt: The pH value of a neutral salt is almost equal to 7.
- Acidic salt: The pH value of an acidic salt is less than 7.
- Basic salt: The pH value of a basic salt is more than 7.
Preparation and uses of important compounds
Caustic Soda (Sodium Hydroxide, NaOH)
Preparation: In the process of electrolytic decomposition of brine (aqueous solution of sodium chloride), brine decomposes to form sodium hydroxide.
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
In this process, chlorine is obtained at anode and hydrogen gas is obtained at cathode as by products. This whole process is known as Chlor – Alkali process.
Uses:
Sodium hydroxide is used for degreasing of metals, manufacturing of paper, soap, detergents, artificial fibres, etc.
Bleaching Powder (Calcium Oxychloride, CaOCl2)
Preparation: Bleaching powder is produced by the action of chlorine on dry slaked lime [Ca(OH)2].
Ca(OH)2 + Cl2→ CaOCl2 + H2O
Uses:
Bleaching powder is used –
(i) for bleaching cotton and linen in the textile industry, for bleaching wood pulp in paper factories and for bleaching washed clothes in laundry;
(ii) as an oxidising agent in many chemical industries; and
(iii) to make drinking water free from germs
Baking Soda (Sodium Hydrogen Carbonate, NaHCO3)
Preparation:
The chemical name of the compound is sodium hydrogen carbonate (NaHCO3). It is produced by the reaction of brine with carbon dioxide and ammonia. This is known as Solvay process.
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
Ammonium Sodium
chloride hydrogen carbonate
Uses:
(i) Baking soda is used in making of baking powder, which is used in cooking.
Baking powder is a mixture of baking soda (sodium hydrogen carbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place –
NaHCO3 + H+ → CO2 + H2O + Sodium salt of acid
(From any acid)
Carbon dioxide produced during the reaction can cause bread or cake to rise making them soft and spongy.
(ii) Baking soda (sodium hydrogen carbonate) is also an ingredient in antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.
(iii) It is also used in soda-acid fire extinguishers.
Washing Soda (Sodium Carbonate, Na2CO3.10H2O )
Preparation:
Sodium carbonate is manufactured by the thermal decomposition of sodium hydrogen carbonate obtained by Solvay process.
NaCl + NH3 + H2O + CO2 → NaHCO3 + NH4Cl
NaHCO3 → Na2CO3 + CO2 + H2O
Heat
Na2CO3 + 10 H2O → Na2CO3.10H2O
Uses:
(i) Sodium carbonate (washing soda) is used in glass, soap and paper industries.
(ii) It is used in the manufacture of sodium compounds such as borax.
(iii) Sodium carbonate can be used as a cleaning agent for domestic purposes.
(iv) It is used for removing permanent hardness of water
Plaster of Paris (Calcium Sulphate Hemihydrate, CaSO4. ½ H2O)
Preparation:
On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate(CaSO4. ½H2O) which is called Plaster of
Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass.
CaSO4. ½H2O + 1½H2O → CaSO4.2H2O
(POP) (Gypsum)
———————————————————————————————————————-
That’s all for Acids Bases and Salts class 10 notes for science chapter 2. We hope it helped you a lot.
If you have any query about the above NCERT class 10 Science notes chapter 2 Acids Bases and Salts, kindly comment down below, we will try our level best to solve that for you.