Chemical reactions: A chemical reaction is a process in which new substances (products) with different properties are formed as a result of the breaking of bonds in reactants.
Chemical equation: A chemical equation is a symbolic representation of a chemical reaction where the reactants and the products are shown by their symbols or formulas.
For example:
- 2Mg(s) + O2(g) → 2MgO(s)
- Na + Cl → NaCl
Ractants: The substances which take part in a chemical reaction are called Reactants.
Products: The new substances produced as a result of a chemical reaction are called products.
Characteristics of Chemical Reactions :
(i) Evolution of gas
(ii) Change in Colour
(iii) Change in state of substance
(iv) Change in temperature
(v) Formation of precipitate
Balanced chemical equation:
The chemical equation in which the number of atoms of each element in the reactants side is equal to that of the products side is called a balanced chemical equation.
Example: Zn + H2SO4 → ZnSO4 + H2.
*According to the Law of Conservation of Mass, mass can neither be created nor destroyed in a chemical reaction. To obey this law, the total mass of elements present in reactants must be equal to the total mass of elements present in products
Unbalanced Chemical Equation:
If the number of atoms of each element in reactants is not equal to the number of atoms of each element present in the product, then the chemical equation is called Unbalanced Chemical Equation.
Example: Fe + H2O → Fe3O4 + H2
Types of chemical reactions
(i) Combination: The reaction in which two or more reactants combine to form one product is called Combination Reactions.
For example:
Mg(s) + O2(g) → 2MgO(s)
(Magnesium) (Oxygen) (Magnesium Oxide)
(ii) Decomposition: The reaction in which one compound decomposes in two or more compounds or elements is known as Decomposition Reaction.
For Example:
- Thermal decomposition: 2Pb(NO2)2 → 2PbO + 4NO2 + O2
- Electrolysis: 2H20 → 2H2 + O2
- Photochemical reaction: 2AgBr → 2Ag + Br2
(iii) Displacement: The chemical reaction in which a more reactive element displaces a less reactive element from a compound is known as Displacement Reaction. It is also known as Substitution Reaction.
For Example:
When zinc reacts with copper sulphate, it forms zinc sulphate and copper metal.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
(iv) Double displacement: The Reaction in which ions are exchanged between two reactants forming new compounds is called Double Displacement Reaction.
For Example: When barium chloride reacts with sodium sulphate, white precipitate of barium sulphate is formed along with sodium chloride.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl (aq)
(v) Precipitation: The reaction in which an insoluble substance (precipitate) is formed by the mixing of two solutions containing salts is called the Precipitation Reaction.
For Example:
When barium chloride reacts with sodium sulphate, white precipitate (ppt.)of barium sulphate is formed along with sodium chloride.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) (white ppt.)+ 2NaCl (aq)
(vi) Neutralization: The reaction in which an acid reacts with a base to form salt and water by an exchange of ions is called Neutralization Reaction.
For example:
When sodium hydroxide reacts with hydrochloric acid, action of both gets neutralised forming sodium chloride and water.
NaOH(aq) + HCl(aq) → NaCl (aq) + H2O (l)
(vii) Oxidation: A reaction that includes gain of oxygen or removal of hydrogen (or loss of electrons).
For example: C + O₂ → CO₂
Reduction: A reaction that includes removal of oxygen or addition of hydrogen (or gain of electrons).
For example:
- A substance that gains oxygen during a reaction, it is said to be oxidised.
- A substance that loses oxygen during a reaction, it is said to be reduced.
Oxidising agent :
- The substance which gives oxygen for oxidation is called an Oxidizing agent.
- The substance which removes hydrogen is also called an Oxidizing agent.
Reducing agent:
- The substance which gives hydrogen for reduction is called a Reducing agent.
- The substance which removes oxygen is also called a Reducing agent.
Chemical Reactions and Equations class 10 notes
Effects of oxidation reaction in everyday life
Corrosion: The gradual eating up of metals by the action of air and moisture on their surface is called corrosion.
The most common example of corrosion is rusting of iron.
4Fe + 3O2 + 2nH2O → 2Fe2O3.nH2O
Steps to prevent rusting of iron:
(i) Coating the iron surface with oil, grease or paint.
(ii) Depositing a layer of zinc on the surface of iron. This process is called galvanisation.
(iii) Forming alloys of iron.
Rancidity: When fats and oils are exposed to air, they get oxidized and become rancid and their smell and colour change. This phenomenon is called rancidity.
Steps to prevent rancidity:
(i) Storing food materials in air-tight containers.
(ii) Packaging food items in bags containing nitrogen.
(iii) Refrigeration of food items.
(iv) Addition of antioxidants or preservatives to the foods containing fats and oils
Activity series
polyatomic Ions
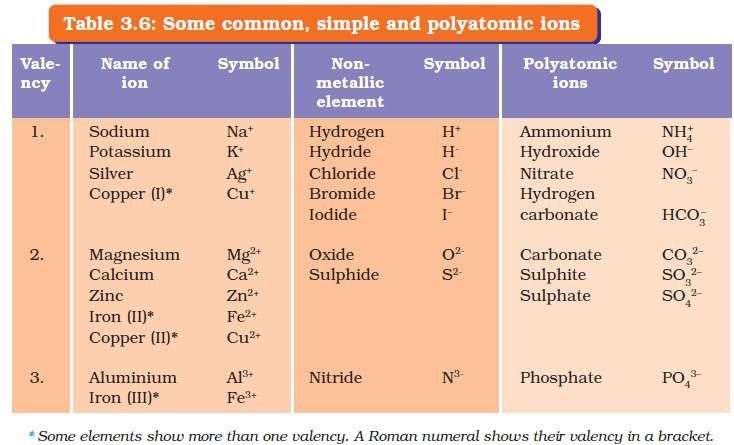
Class 10 Science Solutions
- Chemical Reaction and equations
Class 10 science mcq questions
Click on the button for Class 10 science mcq questions test online and get to know your progress.
